In chemistry, van der Waals' forces are a type of intermolecular force which is a relatively weak force that holds molecules together. Van der Waals' forces are the weakest type of intermolecular force. They are named after the Dutch scientist Johannes Diderik van der Waals (1837–1923). Negatively charged electrons orbit molecules or ions. The electrons create slightly different charges from one end of the molecule to the other. These slight differences are called partial charges, as δ- or δ+. The van der Waals forces are mainly represented in three types of forces and they are Keesom force, Debye force, and London dispersion force.
- Keesom Force
The first contribution to van der Waals forces is due to electrostatic interactions between charges (in molecular ions), dipoles (for polar molecules). It is termed the Keesom interaction, named after Willem Hendrik Keesom. These forces are permanent-permanent dipole interactions which originate from the attraction between permanent dipoles (dipolar molecules) and are temperature dependent.
- Debye Force
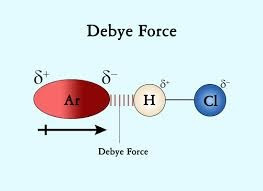
The second contribution is the induction (also termed polarization) or Debye force, arising from interactions between rotating permanent dipoles and from the polarizability of atoms and molecules (induced dipoles). These induced dipoles occur when one molecule with a permanent dipole repels another molecule's electrons. A molecule with permanent dipole can induce a dipole in a similar neighbouring molecule and cause mutual attraction. Debye forces cannot occur between atoms. The forces between induced and permanent dipoles are temperature independent.
One example of an induction interaction between permanent dipole and induced dipole is the interaction between HCl and Ar. In this system, Ar experiences a dipole as its electrons are attracted (to the H side of HCl) or repelled (from the Cl side) by HCl. The induction-interaction force is far weaker than dipole–dipole interaction, but stronger than the London dispersion force.
- London Dispersion Force
London dispersion forces, named after the German-American physicist Fritz London. The third and dominant contribution is the dispersion or London force (fluctuating dipole–induced dipole), which arises due to the non-zero instantaneous dipole moments of all atoms and molecules without any permanent dipoled. Such polarization can be induced either by a polar molecule or by the repulsion of negatively charged electron clouds in non-polar molecules. Thus, London interactions are caused by random fluctuations of electron density in an electron cloud. An atom with a large number of electrons will have a greater associated London force than an atom with fewer electrons.
London dispersion forces are also known as 'dispersion forces', 'London forces', or 'instantaneous dipole–induced dipole forces'. The dispersion (London) force is the most important component because all materials are polarizable, whereas Keesom and Debye forces require permanent dipoles. The London interaction is universal as it is present in atom-atom interactions as well.



Comments
Post a Comment